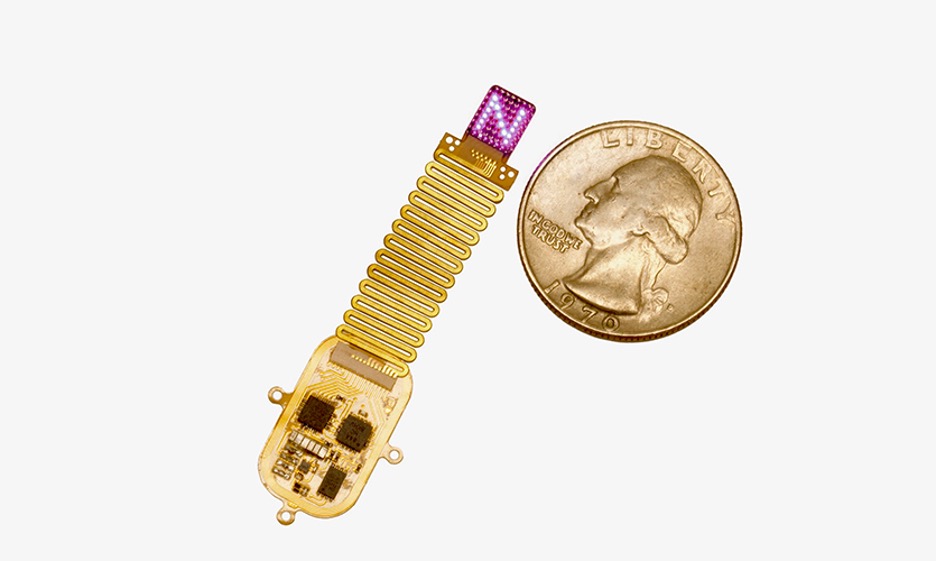
Maxim Integrated's new health sensor platform 3.0 (HSP 3.0) reduces the development time of healthcare wearables by at least six months. Also known as MAXREFDES104#, this ready-to-wear wrist form factor reference design monitors blood oxygen saturation (SpO2), electrocardiogram (ECG), heart rate (HR), body temperature and motion. Included algorithms provide HR, heart-rate variability (HRV), respiration rate (RR), SpO2, body temperature, sleep quality and stress level information at clinical-grade levels. It allows wearable designers to start collecting data immediately, saving at least six months over building these devices from scratch. Designed for wrist-based form factors, HSP 3.0 can be adapted for other dry electrode form factors such as chest patches and smart rings.
Read more: Maxim’s MAXM86146 Is The Thinnest Optical Sensor Solution For Health and Fitness Wearables
MAXREFDES104# can enable end solutions to monitor cardiac heart and respiratory issues for management of ailments like chronic obstructive pulmonary disease (COPD), infectious diseases (e.g. COVID-19), sleep apnea and atrial fibrillation (AFib). Compared to its predecessor, the narrower form factor and enhanced optical architecture of HSP 3.0 improves signal acquisition quality and uses upgraded microcontroller, power, security and sensing ICs. The reference design includes complete optical and electrode designs, along with algorithms to meet clinical requirements, according to a press release.
HSP 3.0 includes the following sensor, power management, microcontroller and algorithm products:
- MAX86176: Lowest-noise optical photoplethysmography (PPG) and electrical ECG analog front end (AFE), which offers 110dB signal-to-noise ratio (SNR) to add SpO2 saturation capability and over 110dB common mode rejection ratio (CMRR) for dry electrode ECG applications. The device enables synchronous acquisition of PPG and ECG measurements, even with independent sample rates, providing pulse transit time for cardiac health use cases.
- MAX20360: Highly integrated power and battery management power management IC (PMIC) optimized for advanced body-worn health sensing devices. It includes Maxim's high-accuracy ModelGauge m5 EZ fuel gauge, a sophisticated haptic driver, and a unique low-noise buck-boost converter that maximizes SNR and minimizes power used for optical bio-sensing.
- MAX32666: Bluetooth (BLE)-enabled, ultra-low-power microcontroller with two Arm Cortex-M4F cores and an additional SmartDMA which permits running the BLE stack independently, leaving the two main cores available for major tasks. Moreover, the microcontroller integrates an entire security suite and error-correcting code (ECC) on the memories to significantly increase the system's robustness.
- MAX32670: Ultra-low-power microcontroller dedicated to Maxim Integrated's world-class PPG algorithms of pulse rate, SpO2, HRV, RR, sleep quality monitoring and stress monitoring. It can be configured either as a sensor hub to support firmware and algorithms or as an algorithm hub to support multiple algorithms. The MAX32670 seamlessly enables customer-desired sensor functionality, including managing the MAX86176 PPG and ECG sensor AFE as well as delivering either raw or calculated data to the outside world.
- MAX30208: The low-power, high-accuracy digital temperature sensor comes in a small package size of 2mm x 2mm. It has 33 percent lower operating current compared to the closest competitive solution. It reads the temperature on the top of the package and can be mounted on a flex cable or PCB, making it easier to design into wearables. With an accuracy of 0.1-degrees Celsius, the MAX30208 meets clinical temperature requirements.
Read more: How to Enhance Reliability of PPG Data for Health Wearables, According To Maxim Integrated
Key Advantages Include
- Faster Time to Market: Saves at least six months in development time
- Clinical-Grade: Accuracy meets regulatory requirements for SpO2 and ambulatory ECG (IEC 60601-2-47)
- Covers Key Vital Signs: Addresses the needs of advanced health wearables with SpO2, ECG, HR, HRV, RR, body temperature and motion
- Complete Reference Design: Empowers designers to innovate with complete access to source code and design files