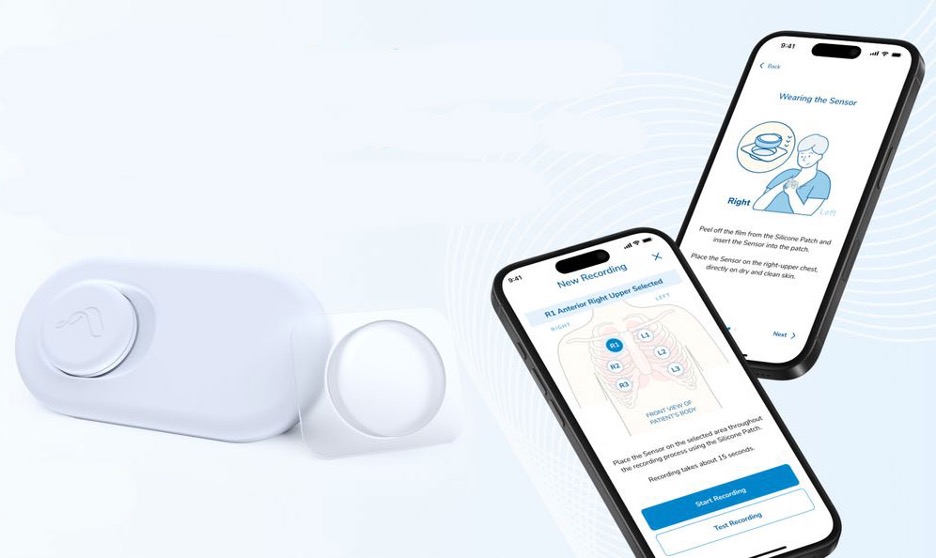
The Therapeutic Goods Administration in Australia has given Singaporean firm Aevice Health permission to use its AI-powered respiratory monitoring system in people three years of age and older.
According to a press release from Aevice, this is the nation's first approval of a device that combines digital auscultation, heart and respiratory rate monitoring, and wheeze detection into a single Class IIa medical equipment for patients older than three years.
The AeviceMD was also added to the Australian Register of Therapeutic Goods as a result of the approval. CEO Adrian Ang told Mobihealth News that the firm was given accelerated registration since the TGA recognizes Singapore's Health Sciences Authority as a Comparable Overseas Regulator, which gave it its first regulatory approval. "Aevice Health has leveraged this pathway for market access into Australia," he stated.
The results of Aevice's observational study with National University Hospital in Singapore, which demonstrated that the AI-powered wearable stethoscope achieved 92% detection accuracy in a pediatric population, were also highlighted by the Australian regulator.
Related AI-Powered Stethoscope Detects Heart Disease
Further, the wearable system pairs with mobile and web applications which are expected to enable clinicians to monitor patients outside traditional healthcare settings.
This approach seeks to reduce the need for frequent in-person visits while supporting timely intervention when symptoms change.
The new approval in Australia follows its prior regulatory clearance from the U.S. Food and Drug Administration for pediatric use of the same wearable stethoscope.
Commenting on the new grant, CEO Ang said, Aevice Health, “With its new TGA clearance, Aevice will explore potential pilot deployments and workflow integrations with local hospitals, respiratory specialists, and clinical teams. These discussions will focus on incorporating respiratory monitoring into existing asthma management pathways in the outpatient care model for their remote patient or therapeutic monitoring programs.”


